Biological Use Authorization (BUA)
The Biological Use Authorization (BUA) application is submitted by Principal Investigators to document the scope of their teaching or research projects. Delegates may initiate and fill out the application but only Principal Investigators (PIs) who meet the criteria for PI eligibility as defined in UCR Policy 527-3 can submit.
Whom can I contact?
Environmental Health & Safety
UCR Biological Safety Officer
ehsbiosafety@ucr.edu
951-827-5528
How do I submit a BUA application?
Log into https://app.riskandsafety.com
To submit an amendment or renewal to a previously approved BUA
- Your previously approved BUA(s) has been migrated over from the old BUA Portal. The BUAs are listed under your Workspace.
- Click on the BUA you wish to amend or renew.
- On the right hand column, select Amendment or Renewal.
- Review and edit each section as appropriate then click on Submit to Biosafety Review.
To submit a new BUA
- On the right hand side under Quick Links, select Begin a Biological Use Authorization.
- Answer and input information for all biological materials that you plan to work with. Fields with the red * are required and must be filled out.
- Depending on the materials you plan to work with, upload any required Standard Operating Procedures (SOPs) in the appropriate fields. Templates for the SOPs are linked within the BUA form.
- Fill in and edit each section as appropriate then click on Submit to Biosafety Review.
Tutorials
Tutorial story on how to submit new, amendment, and renewal BUAs for PIs. Includes the BUA approval process and how the IBC members and Chair access BUAs to review and approve them. Questions about the BUA process should be submitted to ehsbiosafety@ucr.edu.
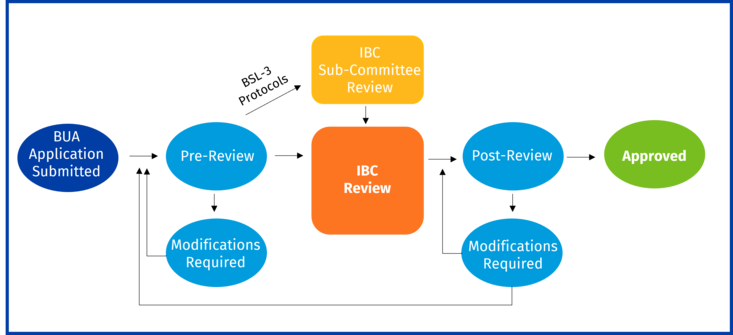
What is the BUA review and approval process?
All submitted BUAs are pre-reviewed by the IBC Administrator/EH&S (Biosafety Review) to ensure completeness of submitted information. The IBC Administrator will return the BUA to the PI if additional information is required to address any issues.
- If a BUA is determined to be exempt from IBC oversight, the IBC Administrator will notify the PI of this status. No additional action is required.
- Once the BUA passes Biosafety Review, it will go to full IBC Review or sub-committee review (BSL-3 protocols only) prior to going to full IBC review.
- Post review, the PI will be notified if modifications are required or if the BUA has received approval.
Additional compliance reviews that may be required:
Dual Use Research of Concern (DURC): DURC review life sciences research that can be reasonably anticipated to provide knowledge, information, products, or technologies that could be intentionally misused to pose a significant threat, with broad potential consequences, to public health and safety, agricultural crops and other plants, animals, the environment, materiel, or national security. This applies to 15 agents and 7 sets of experimental effects of concern.
Institutional Animal Care and Use Committee (IACUC): The use of vertebrate animals (e.g., testing viable recombinant or synthetically modified microorganisms on whole animals) must be approved by the IACUC (via an AUP).
Institutional Review Board (IRB): IRB review of human protections may be required for some activities, such as the collection of blood or primary tissues.
Stem Cell Research Oversight (SCRO): SCRO review of the use or storage of human pluripotent stem cells is required.
Material Transfer Agreement (MTA): Some biohazardous materials (e.g., plasmids, vectors, viruses, human cell lines) cannot be obtained without a Material Transfer Agreement (MTA). The Research and Economic Development Office is required to review these contracts in accordance with UC mandated restrictions. It is recommended that you contact mta@ucr.edu as soon as you have determined you will be obtaining materials from an external source.
Required Training
BUA approval is contingent upon completion of required trainings. All personnel that may be present in the laboratory, whether they directly work on the project or not, must complete the required trainings. This is to ensure that any personnel who may potentially be exposed to the proposed work materials have received adequate training. The required trainings are:
- Biosafety (must be completed once)
- Laboratory Safety Fundamentals (every 3 years). Note: Safety Orientation does not fulfill the requirement for Laboratory Safety Fundamentals
If the proposed project works with the following materials, additional trainings required are:
- Bloodborne Pathogens Online (annually) (for projects working with with human/non-human primate materials or other potentially infectious materials)
- Aerosol Transmissible Diseases (annually) (for projects working with aerosol transmissible diseases or pathogens – laboratory as listed in the Aerosol Transmissible Diseases Standard
All trainings can be found in the UC Learning Center: https://ucrlearning.ucr.edu/.