Institutional Review Entity (IRE)
The Institutional Review Entity (IRE) functions as the UCR body responsible for review and oversight of research that falls within the scope of the 2024 United States Government Policy for Oversight of Dual Use Research of Concern (DURC) and Pathogens with Enhanced Pandemic Potential (PEPP) (“Policy”). The IRE Committee is administered by Environmental Health & Safety (EH&S) while membership is appointed by the Vice Chancellor of Research and Economic Development (VCRED).
Effective May 6, 2025, the Policy addresses oversight of research on biological agents and toxins that, when enhanced, have the potential to pose risks to public health, agriculture, food security, economic security, or national security. It consolidates and replaces the 2012 Federal DURC Policy, the 2014 Institutional DURC Policy, and the 2017 P3CO Framework, creating a single, streamlined framework for governance.
-
Scope
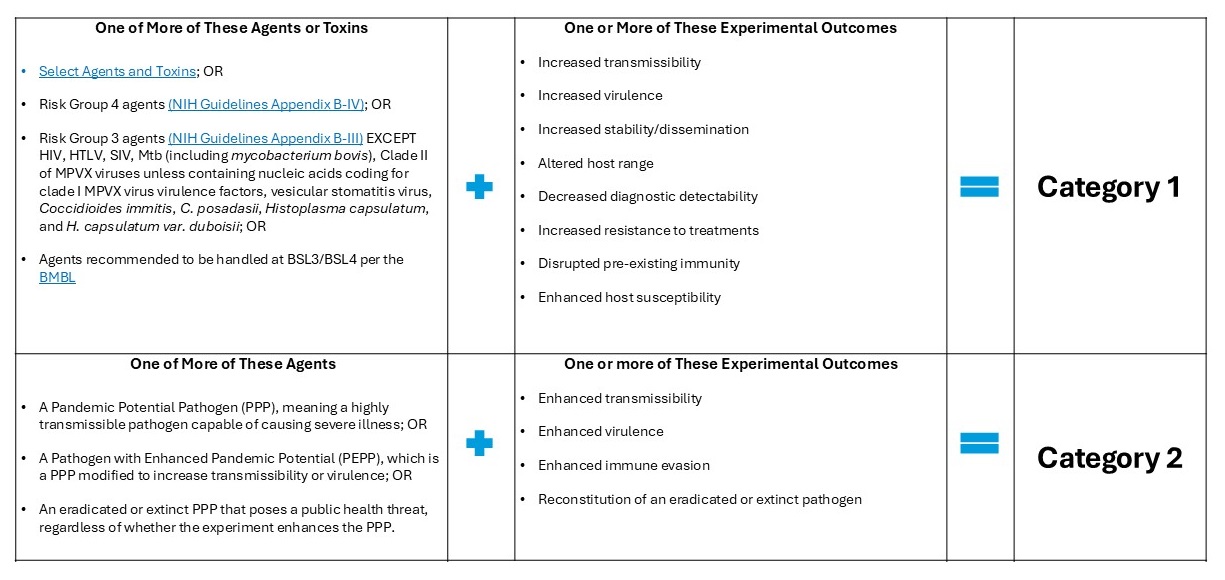
The Policy covers research that falls within 2 categories as outlined below. Category 1 research involves 1 or more of the agents or toxins listed below and is reasonably expected to result in one or more of the listed experimental outcomes. Category 2 research involves or is reasonably anticipated to result in a pathogen with pandemic potential and is reasonably expected to result in one or more of the listed experimental outcomes.
-
Definitions
Dual Use Research of Concern (DURC) – Life sciences research hat, based on current understanding, can be reasonably anticipated to provide knowledge, information, products, or technologies that could be misapplied to do harm with no, or only minor, modification to pose a significant threat with potential consequences to public health and safety, agricultural crops and other plants, animals, the environment, materiel, or national security.
Federal Funding Agency (FFA) – The FFA is a federal department, agency, institute, center, or office that funds or sponsors intramural or extramural research at research institutions in the United States or internationally, with biological agents or toxins where the research is within Category 1 or Category 2 under the Policy.
Institutional Contact for Dual Use Research (ICDUR) – The ICDUR is the official designated by the research institution to serve as an internal resource for application of this Policy as well as the liaison (as necessary) between the institution and the relevant federal funding agency.
Pathogen with Enhanced Pandemic Potential (PEPP) – Type of pathogen with pandemic potential (PPP) resulting from experiments that enhance a pathogen’s transmissibility or virulence, or disrupt the effectiveness of pre-existing immunity, regardless of its progenitor agent, such that it may pose a significant threat to public health, the capacity of health systems to function, or national security. Wild-type pathogens that are circulating in or have been recovered from nature are not PEPPs but may be considered PPPs because of their pandemic potential.
Pathogen with Pandemic Potential (PPP) – Pathogen that is likely capable of wide and uncontrollable spread in a human population and would likely cause moderate to severe disease and/or mortality in humans.
-
Responsibilities
Principal Investigators
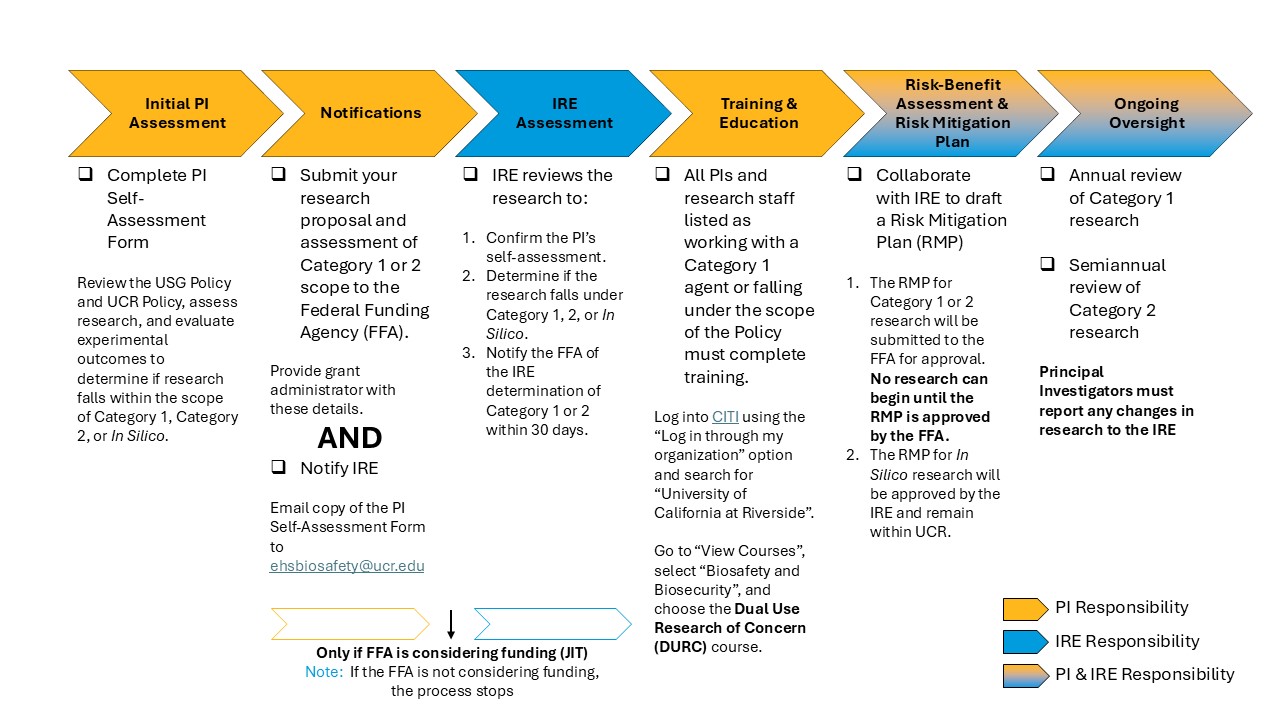
PIs are responsible for conducting a self-assessment to determine if their research falls within Categories 1 and/or 2 of the Policy, notifying the FFA and IRE if it does, and collaborating with the IRE to develop and implement a Risk Mitigation Plan (RMP).
Institutional Review Entity
The IRE shall ensure that no research classified as Category 1 or Category 2 is conducted unless the PIs have completed required the notifications, submissions, and training as outlined in the Policy. The IRE will collaborate with PIs to perform the risk-benefit assessment and develop a risk mitigation plan, if necessary. In addition, the IRE will conduct ongoing monitoring and review of active risk mitigation plans and research to ensure compliance with the Policy to address evolving risks or changes in research scope.
-
DURC/PEPP Review Process
Principal Investigators (PIs) are responsible for completing a self-assessment to determine if their research falls within the scope of the Policy, notifying the funding agency and IRE of said assessment, and collaborating with the IRE to develop and implement a risk mitigation plan if their research is determined to fall within the Policy.
-
Resources
- PI Self-Assessment
- Risk Mitigation Plan Template
- Implementation Guidance for USG Policy for Oversight of Dual Use Research of Concern and Pathogens with Enhanced Pandemic Potential
- PI Self-Assessment